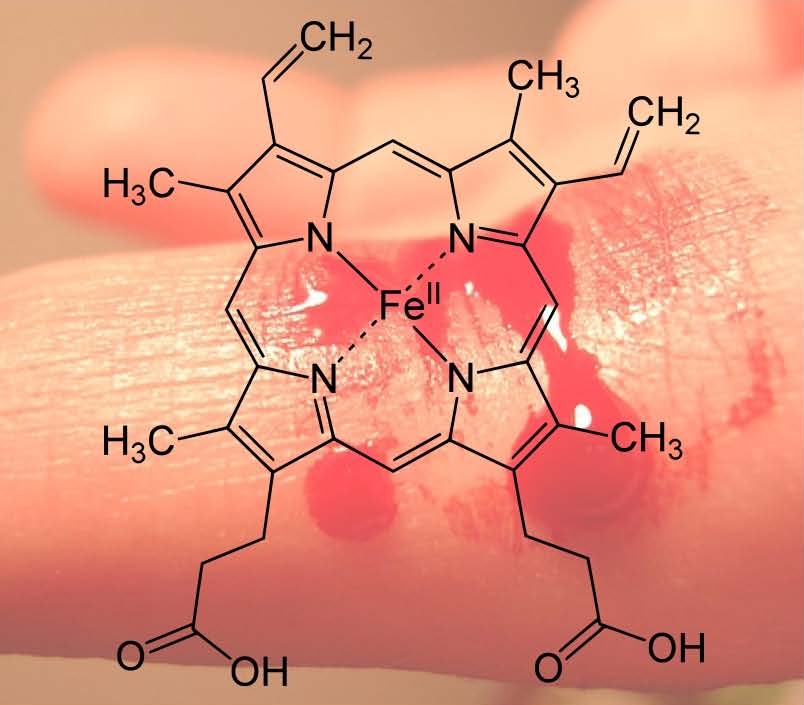
Why is blood red? It contains heme, a molecule which is very similar to chlorophyll... but with iron instead of magnesium at its heart!
Like chlorophyll, heme is a porphyrin. That means it's made of four pentagons of carbon and nitrogen joined together by bridges of carbon. In chlorophyll a, almost all the carbons connected by alternating single and double bonds. In heme, they all are.
These alternating single and double bonds let electrons roam around in a smeared-out, quantum way... and when light hits the molecule, it's like a kid jumping on a trampoline! It makes these smeared-out electrons vibrate back and forth. But unlike the kid, the light gets absorbed if it's vibrating at the right frequency: it transfers all its energy to the molecule.
Chemists have a really sexy way of talking. Listen to how they say what I just said:
Heme and chlorophyll are porphyrins. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH-). Porphyrins are aromatic. That is, they obey Hückel's rule for aromaticity, possessing 4n+2 π electrons (where n is the number of carbon atoms) delocalized over the macrocycle. Thus porphyrins are highly conjugated systems. As a consequence, they typically have intense absorption bands in the visible region.
Nothing about kids jumping on trampolines...
But I've got some questions. It's important that chlorophyll absorbs light - that's how plants get energy. It doesn't seem important that heme absorbs light. What heme does is grab oxygen, carry it to where it needs to go, and then let it go. So:
Puzzle 1. Is it just a 'coincidence' that blood is red, or is its color somehow a necessary aspect of its biological function?
Puzzle 2. Did heme evolve from chlorophyll? Did chlorophyll evolve from heme? Did they both evolve from a common molecule? Or in each case did nature arrive separately at the idea of using a porphyrin with a metal ion in the middle?
I can give you some hints for Puzzle 2. Hemoglobin and hemoglobin-like molecules are also found in many invertebrates, fungi, and plants. These molecules can carry oxygen, or transport and regulate other things such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide ions.
There are also porphyrins containing other metals! For example, the blood of crabs contains hemocyanin, which has copper instead of iron.
The molecule shown above is heme b, the most common form in humans. Heme a is a lot like chlorophyll a — they both have a long chain of carbons attached to the porphyrin. Here is heme a:
and here is chlorophyll a:
The portion with the bracket around it is repeated twice: that's what the subscript 2 means.
For some attempts to answer my puzzles, read the comments on my Google+ post.
Why are carrots orange? Because they contain carotene.
Carotene comes in different forms, but here is one of the most common: β-carotene. I like it because it's perfectly symmetrical. It has a long chain of carbons with alternating single and double bonds. Electrons vibrating along this chain absorb blue light. So the carrots have the opposite color: orange!
Humans need a chemical called retinal in order to see:
It looks roughly like half a carotene molecule — and like carotene, it's good at absorbing light. Attached to a larger protein molecule called an opsin, retinal acts like a kind of antenna, catching particles of light.Humans can't produce retinal without help from the foods we eat. Any chemical we can use to produce retinal is called vitamin A. So vitamin A isn't one specific chemical: it's a group. But beta carotene counts as a form of vitamin A.
So, you can do something amazing without even thinking about it: you can eat a carrot, extract the beta carotene, break it in half, turn it into retinal, attach it to an opsin, and see! That's why eating carrots is good for your vision.
It's no coincidence that plants contain carotene: its light-absorbing abilities play a role in photosynthesis. But...
Puzzle 1. Why do carrots, which are underground, contain a lot of carotene?
Puzzle 2. Why are oranges orange?
You can see alpha-, beta-, gamma- and delta-carotene here:
For answers to the puzzles, see my Google+ post.
Look at this amazing molecule! It looks a bit like chlorophyll or heme, but it's got an atom of cobalt in the middle where those others have magnesium or iron.
What's amazing is not that this molecule exists. What's amazing is that you need to eat it — or something very like it. It's called vitamin B12, and you need about 2 micrograms a day.
In the middle ages, German miners called some rocks kobold ore — German for 'goblin' ore — because they were poor in known metals and gave poisonous fumes when you heated them. In 1739 a Swedish chemist named Georg Brandt discovered that these rocks contained a new metal. So this metal, cobalt, is named after goblins!
It took a lot longer to realize that cobalt is essential for human life. In 1934, three chemists won the Nobel prize for discovering that eating liver could prevent a disease called pernicious anemia. The crucial chemical in liver — now called vitamin B12 — was isolated in 1947. But its structure was only understood in 1956.
Animals can't make vitamin B12 — only bacteria, and a kingdom of older life forms called archaea, can do it. But animals store it, so if you eat liver, or other kinds of meat, or fish, or eggs or milk, you'll get vitamin B12. And you don't need very much!
The molecule here is a form of vitamin B12 called cyanocobalmin, because it has a cyanide ion — CN — attached to the cobalt. This is an artificial form of B12 you can buy at a drug store.
Puzzle. What is vitamin B11?
For the answer and some nice discussions see my Google+ post.
June 7, 2014
Three days ago my wife Lisa started seeing flashes like lightning in
the peripheral vision of her right eye. Then she started seeing black
specks like gnats. Years ago she nearly had a detached retina, which
doctors bolted down with laser surgery. So she has always been on the
lookout for symptoms like this.
We went to the emergency room around 8 pm, shortly after the black specks formed a 'curtain' in the middle of her field of vision. Standing two yards from a mirror, she said: "I can't even see the color of my eyes."
In some ways we're all waiting for something like this to happen.
We're all going to die... or at least, most of us: some 'transhumanists' optimistically freeze their brains in hopes that future generations will revive them, but even if this hope comes true, most people can't afford that. I've gotten used to the idea of dying, so I'm not going to extraordinary lengths to prevent it. The harder part is slowly walking down the stairway of old age: getting used to worse and worse health, slower wits, less energy... down to nothing.
It's a journey of renunciation. Wise old people don't talk about this much, because they know it annoys and (secretly) upsets the young. It's better to let them live in their happy self-absorbed world: no point in spoiling it.
This must be one reason people like having children and grandchildren: as you falter and fade, they (with luck) are still growing stronger. The spotlight nicely shifts from you to them... so when death pulls you off the stage with its hook, nobody pays much attention: overall, the show is still a happy one.
Since I don't have children, I don't know exactly what this feels like. I tend to use math and physics to create that happy dreamworld where everything keeps getting better and better... though I also have students, who will carry on when I conk out.
When I switched from pure math and fancy theoretical physics and started thinking hard about global warming, I had to accept the extra emotional burden of facing a world that was not all bright and beautiful. I think some of my fans left at this point: it turns out they wanted my science explanations to cheer them up! But I've got a lot of built-in pep and happiness, so I don't need a diet of pure candy.
Anyway, it seems that Lisa had a posterior vitreous detachment, where the vitreous membrane separates from the retina. It's not a disaster: three quarters of people over 65 get this condition! Here eye bled a bit when this happened, so she has a bunch of red blood cells floating in her vitreous humor. Supposedly in a month these blood cells will go away, decomposed somehow by the magically self-repairing body.
So, it's not so bad. I know that this is just one more step down that spiral stairway to darkness that Lisa and I are walking, hand in hand. But that's just how it goes.
Lisa seems less perturbed by this than me: while I'm writing this little essay, she's packing her suitcases. At 9 tonight she's taking a shuttle to the airport and then flying to Singapore! I'm going to a conference in Banff for a week, on computation with chemical reaction networks. Then I'll join her in Singapore, where we will spend the summer working.
So life goes on. Until it doesn't. And even then, it goes on.
This beautiful molecule looks a lot like heme, but with zinc instead of iron at its heart! If you get lead poisoning, your blood will start getting this instead of heme in it.
This molecule is called zinc protoporphyrin. It can show up in your blood for several reasons, including:
Puzzle 1. Why?
I don't know! I guess it makes sense that if you have an iron deficiency the body might wind up grabbing some similar metal and using it as a substitute for iron in the heme.
Puzzle 2. Does it work? In other words, can it actually help you breathe?
Anyway, people use the presence of zinc protoporphyrin as a test for lead poisoning and other problems. Zinc blood is a bad sign.
This molecule looks like chlorophyll or heme — but where those have magnesium or iron, this has an atom of nickel at its heart! And this molecule, called cofactor F430, is part of why cows burp so much.
Like all vertebrates, cows can't break down cellulose, the main ingredient in grass. So they have 4 stomachs, and in the 'rumen' there are trillions of tiny organisms that can break down cellulose. These organisms make sugars, which the cows can digest — but also other chemicals, including methane. The cows fart and burp... and since methane is a powerful greenhouse gas, about 40% of all global warming due to agricultural activity comes from this process! Not just from cows, but also sheep and goats.
The tiny organisms that make methane are called methanobacteria... but now we know they aren't really bacteria. We've learned a lot about the tree of life in recent decades. Now life is classified into three huge 'domains': Archaea, Bacteria, and Eukaryota. You, the cow, and the grass are all in Eukaryota. Archaea are a very ancient domain, and they include methanobacteria.
Here's something cool: some Archaea called methanotrophs use cofactor F430 in reverse to metabolize methane instead of make it. Could this go back to the days when Earth's atmosphere had lots of methane? Now these methane-eaters live in mud, marshes, soils, rice paddies, landfills and oceans. If we learn to use them, we could use them to help fight global warming!
A Thorne–Żytkow object is a giant star that has swallowed a neutron star. The idea of such a thing was invented by Kip Thorne and Anna Żytkow in 1976. Now we may have found one!
A Thorne–Żytkow object can be formed when a neutron star collides with a red giant or supergiant star. They might simply collide by accident... but there's a lot of room in space, so this is very unlikely.
More likely, the neutron star and the giant could be part of a binary star system! A neutron star forms when a giant star runs out of fuel, collapses, and explodes as a supernova, blowing off its outer layers... while the core crushes down to a ball of neutronium. So, you have to image one giant star in a binary system doing this, while its partner survives.
Then, they might slowly spiral down due to friction. A red giant is usually surrounded by a lot of very thin hot gas. Once the neutron star enters this, drag will make the two stars spiral toward each other more quickly. Depending on their initial separation, this process could take hundreds of years. When the two finally collide, the neutron star and the core of the giant star will merge.
If their combined mass is big enough, the two will then collapse into a black hole, resulting in a supernova that blasts away the outer layers of the giant star. Otherwise, we'll get a giant star with a neutron star at its core: a Thorne–Żytkow object!
Strange things could happen in a Thorne–Żytkow object. As gas falls from the giant onto the surface of the neutron star, it would get very hot — about a billion kelvin — thanks to fusion and compression due to gravity.
Under these conditions, the rapid proton capture process or rp-process might occur! This happens when protons keep smacking into an atomic nucleus, making it heavier and heavier. This can only happen when it's very hot, since protons are strongly repelled by the positive charge of the nucleus.
There are a few places in the universe where the rp-process can occur — not just Thorne–Żytkow objects. A more common option would be a binary system with one neutron star and one ordinary star, where gas sucked up by the neutron star gets very hot and creates a big explosion. That's called an X-ray burster.
Some astronomers now claim that the star HV 2112 in the Small Magellanic Cloud is a Thorne–Żytkow object. I wouldn't be as confident as the headline in this story, but it's definitely worth a read:
The picture here is by Mike Guidry, who has a nice webpage on four places where the rp-process could occur:
You can read the original paper by Kip Thorne and Anna Żytkow here:
I now live on the 14th story of an apartment for faculty at the National University of Singapore. In the lobby of each floor there's a door labelled electrical riser, where wires go up from floor to floor. There's a door labelled TAS riser, where telecommunications cables go up. And there's a door labelled ELV riser, where elves go up.
Well, that's what I imagined. But then I looked it up. Much to my surprise, I found that ELV actually stands for electrostatically levitating vampire.
I pondered this for days. Finally, unable to resist, I opened up the ELV riser last evening. I saw black shrouded figures, light as feathers, floating up a large shaft, lifted by the tiny voltage gradient. They congregate in the rooftop garden, drinking cocktails and chatting... and then, when the moon comes up, they launch themselves into the night sky. You can see them sailing with their huge bat-like wings toward the poor neighborhoods of Geylang... the seedy streets where prostitutes and illegal immigrants live.... knowing how unlikely it is for the bored police to do more than file a routine report when yet another victim is found dead in a back alley.
You might think life in faculty housing would be boring, but it's not.
Too cute! Here's a mother pangolin carrying its baby, photographed by Firdia Lisnawati at a zoo in Bali. The baby was born at the zoo on May 31, and this photo was snapped last Thursday when it was less than 3 weeks old.
A pangolin is a kind of scaly anteater found in tropical regions throughout Africa and Asia. A friend of mine even saw one in a park in Singapore! There are eight different species, from the giant pangolin to the tree pangolin. Some are endangered because people eat them or — even worse — kill them for the completely imaginary medical properties of their scales! In one incident in 2013, 10,000 kilograms of pangolin meat was seized from a Chinese vessel that ran aground in the Philippines.
When a pangolin is born, its scales are soft and white; then they gradually get harder and darker. At first the mother stays with the baby in a burrow, nursing it, and she will wrap her body around it if she senses danger. After about a month, the baby first leaves the burrow riding on the mother's back. Weaning takes place at approximately three months of age, and the young pangolin begins to eat insects. At two years of age, the youth is grown up and is left to fend for itself.
Pangolins were originally classified among the Xenarthra, which include ordinary anteaters, sloths, and armadillos. But newer genetic evidence indicates their closest living relatives are in the order Carnivora, which include wolves, bears, tigers, etc. So, they're are now in their own order, Pholidota, next to Carnivora in the tree of life. They've got precious genetic distinctiveness! Hug a pangolin today!
To learn about pangolin trafficking, read:
Are these waves going to the left or the right?
The big pulses are moving to the right. The group velocity is the velocity of the big pulses, so this is positive.
The little wiggles are moving to the left. The phase velocity is the velocity of these little wiggles, so this is negative.
So, the group velocity and phase velocity can be completely different! You have to be careful when you talk about the speed of waves. Indeed, if this wave was a rope wiggling up and down, the rope itself wouldn't be going left or right! The speed of the wave is an abstraction... and we can define it in different ways.
There's also the signal velocity. Say you start with no wave at all to the right of some point. How far to the right of that point can the wave get in one second? That's the signal velocity.
As far as we know, the signal velocity of light is about 299,792,458 meters per second no matter what. That's a law of physics.
But in air, the group velocity and phase velocity of light are less than this. And in glass, the phase velocity of X-rays is more than this! That doesn't violate any laws of physics.
It's less common, but sometimes the group velocity of light is faster than 299,792,458 meters per second. This doesn't violate any laws of physics either!
For a great illustration of faster-than-light group velocities, try this:
The title of this painting by David Fricks is "Neutrino Flux - 1987A"
SN 1987A is the name of a supernova in the Tarantula Nebula of the Large Magellanic Cloud whose light — and neutrinos! — reached us in 1987.
Though it was over 150,000 light-years away, it was visible to the naked eye. Two to three hours before the visible light reached us, a burst of neutrinos was observed at three separate neutrino observatories. They only saw a total of 24 neutrinos... but this is what you'd expect from a supernova that far! It probably put out 1046 joules of energy, 99% in the form of neutrinos... for a whopping total of 1058 neutrinos.
Some went through you, and some went through this monkey.
Here's a little mystery about supernova 1987A. It acted like a type II supernova, where a massive star runs out of fuel and its core suddenly collapses. Given the size of the original star, its core probably crunched down to a neutron star. But so far astronomers haven't been able to find this neutron star!
There are a few possible explanations. The first, and least exciting, is that the neutron star is there, but we can't see it yet because it's still surrounded by dust clouds.
A more exciting option is that lots of stuff fell back on the neutron star and it collapsed into a black hole. We expect a black hole to form when a star more than 20 times as massive as the Sun goes supernova. The star that formed supernova 1987A, a blue supergiant called Sanduleak -69° 202, was apparently close to that borderline.
There are even more exciting options, but it's good to remember that in science, the most exciting possibilities tend to be the least likely.
But it was already a surprise that Sanduleak -69° 202 became a supernova in the first place. Astronomers didn't think that blue supergiants could run out of fuel and go supernova like that! So, maybe more surprises are in store.
I thank David Fricks for emailing me this picture.
© 2014 John Baez
baez@math.removethis.ucr.andthis.edu