Let us look at a simple model of hydrogen atom: we neglect the spin of the proton and the electron, and relativistic effects. What remains is a point charge in an inverse-square force field-- the classical Kepler problem.
With these simplifications, the states of the hydrogen atom can be
specified by giving three integer labels ![]() , with:
, with:
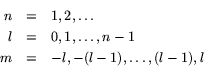

I should make one refinement to this description of the ``old quantum'' viewpoint. The quantum conditions apply only to so-called stationary orbits. Bohr offered no details for what happened during a transition from one stationary orbit to another (a ``quantum jump''), nor could he explain why these orbits were stationary. Initially, physicists probably regarded these questions as topics for future research.
In the post-1925 reformulation, we have to solve a particular instance of
Schrödinger's equation, subject to certain boundary conditions. The
space of all solutions is a Hilbert space, with a basis ![]() .
The vector
.
The vector ![]() is simultaneously an eigenvector of three operators:
is simultaneously an eigenvector of three operators:
![]() , the energy
, the energy
![]() , the magnitude of the orbital angular momentum
, the magnitude of the orbital angular momentum
![]() , the
, the ![]() -component of the orbital angular momentum
-component of the orbital angular momentum
So we can say that if the hydrogen atom is in state ![]() , then it
has a definite energy, and its angular momentum has a definite magnitude
and
, then it
has a definite energy, and its angular momentum has a definite magnitude
and ![]() -component. In fact, the eigenvalues for
-component. In fact, the eigenvalues for ![]() are:
are:
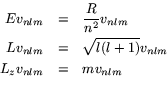
The modern equivalent of the old notion of ``stationary orbit'' is
``eigenstate of the energy operator''. Such eigenstates do not change with
time, and they possess a definite value for the energy. (These facts are
closely related.) Schrödinger's equation, in fact, amounts to
![]() , where
, where ![]() is the energy operator.
is the energy operator.
Figure 1 gives a pictorial representation of the basis
![]() in a form known as a term scheme. The horizontal lines
stand for basis vectors (or equivalently, stationary quantum states);
height gives energy, and transitions are indicated by slanted lines. (Only
three transitions are pictured, to avoid clutter.)
in a form known as a term scheme. The horizontal lines
stand for basis vectors (or equivalently, stationary quantum states);
height gives energy, and transitions are indicated by slanted lines. (Only
three transitions are pictured, to avoid clutter.)
From this simple diagram, many treasures flow. The next few paragraphs give a taste.
© 2001 Michael Weiss